Coping With Loss Of Hospital, Rural Town Realizes: We Don’t Need A Hospital
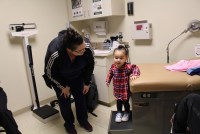
It’s been about a year since the hospital in Fort Scott, Kan., closed. The lessons for this community about meeting its residents’ health needs could provide insights for the rest of the country.
Air Ambulances Woo Rural Consumers With Memberships That May Leave Them Hanging
State regulators and even one medevac company have raised doubts about prepaid subscriptions and promised benefits offered by air ambulance companies.
After A Rural Hospital Closes, Delays In Emergency Care Cost Patients Dearly
The loss of the longtime hospital in Fort Scott, Kan., forces trauma patients to deal with changing services and expectations.
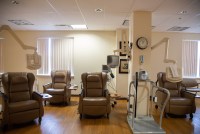
Have Cancer, Must Travel: Patients Left In Lurch After Hospital Closes
As the rural town of Fort Scott, Kan., grapples with the closure of its hospital, cancer patients face new challenges as they try to continue their treatments in different locations.
Robin Hood To Rescue Of Rural Hospitals? New Math Promised On Medicare Payments
A proposed adjustment to the wage index, used in setting a hospital’s Medicare reimbursement payments, could be a lifeline for some rural facilities.
Dealing With Hospital Closure, Pioneer Kansas Town Asks: What Comes Next?
After depending on the local hospital for more than a century, Fort Scott residents now are trying to cope with life without it.
Why The U.S. Remains The World’s Most Expensive Market For ‘Biologic’ Drugs
Biologic drugs, made from living organisms, are big moneymakers partly because they have little competition from “biosimilars.” It’s a very different story in Europe.
For The Asking, A Check Is In The Mail To Help Pay For Costly Drugs
It’s a little-known secret that patients can get thousands of dollars directly from a drugmaker.
Government Investigation Finds Flaws In the FDA’s Orphan Drug Program
A probe by the Government Accountability Office cites breakdowns in the Food and Drug Administration program that approves drugs for rare diseases.
Trump Adds A Global Pricing Plan To Wide Attack On Drug Prices, But Doubts Persist
Over the past five months, the Trump administration has proposed a series of reforms to lower the cost of prescription drugs.
TV Ads Must Trumpet Drug Prices, Trump Administration Says. Pharma Tries A Plan B.
Drug pricing is a top issue in the run-up to the midterm elections.
Drugmakers Play The Patent Game To Lock In Prices, Block Competitors
Pharmaceutical companies like Purdue Pharma, maker of OxyContin, often win patents for incremental changes with debatable value. Now there’s a twist involving an opioid treatment.
Unwitting Patients, Copycat Comments Play Hidden Role In Federal Rule-Making
As HHS decided to cut $1.6 billion in drug payments to hospitals, it weighed thousands of comments generated by a pharmaceutical-funded advocacy group.
Trump Administration Sinks Teeth Into Paring Down Drug Prices, On 5 Key Points
Instead of waiting for congressional action, federal regulators are looking at a series of actions to spur competition and drive down the cost of medicines.
Déjà Voodoo: Pharma’s Promises To Curb Drug Prices Have Been Heard Before
Several major drugmakers vow to contain drug prices, but similar pledges since the 1990s have not had much impact.
Top Policy Expert’s Ties To Giant Drugmaker Often Go Unstated
Dr. Mark McClellan joined Johnson & Johnson’s board of directors after leaving the FDA, but the connection often isn’t mentioned in research papers or public events.
Trump Vows (Again) To Lower Drug Prices But Skeptics Doubt Much Will Change
President Donald Trump’s much-awaited speech about slashing drug costs was long on rhetoric but short on specifics that will reduce prices.
For The Babies Of The Opioid Crisis, The Best Care May Be Mom’s Recovery
Research is just beginning on infants born with neonatal abstinence syndrome, and doctors are optimistic that normal development is possible. Monitoring the families and making sure parents are treated for addiction is key.
Dissecting The Rhetoric Vs. Reality Of Trump’s Tough Talk On Drug Prices
President Donald Trump’s upcoming speech on drug prices comes after months of public comments and debate about tackling the issue.
How A Drugmaker Turned The Abortion Pill Into A Rare-Disease Profit Machine
An abortion drug invented decades ago is being used to treat Cushing’s syndrome — and it’s bringing in tens of millions of dollars a year.