Latest KFF Health News Stories
KHN and California Healthline staff made the rounds on national and local media this week to discuss their stories. Here’s a collection of their appearances.
As Nation Awaits Vaccine, Biden Is Under Pressure to Name New FDA Chief ASAP
It typically takes months to install new leadership, but with COVID deaths set to surge through the winter, many Democrats say Biden doesn’t have that sort of time.
Distrusting Trump, States Plan to Vet COVID Vaccines Themselves. Bad Idea, Say Experts.
California and at least five other states have said they may independently vet any vaccines. Experts warn that could needlessly confuse the public.
Comienzan a popularizarse las pruebas de saliva para COVID, que son fáciles de usar
Ocho meses después del inicio de la pandemia, la prueba de saliva gana adeptos y decenas de miles de personas en todo el país se someten a estas pruebas diariamente.
Easier-to-Use Coronavirus Saliva Tests Start to Catch On
Regulators and scientists have been leery of introducing the tests, preferring to rely on tried-and-true methods, but evidence is mounting that the spit and swab tests may be more convenient and just as accurate.
These Secret Safety Panels Will Pick the COVID Vaccine Winners
Data and safety monitoring boards work under a cloak of secrecy meant to prevent undue influence by stakeholders, such as companies or the government. In the Trump era, some worry the anonymity could actually invite it.
¿Regalo para Florida? Trump aprobaría pronto importación de medicamentos de Canadá
A pesar de las objeciones de las farmacéuticas, se espera que la administración Trump finalice pronto el plan que permitiría a los estados importar medicamentos de venta bajo receta.
Election Gift for Florida? Trump Poised to Approve Drug Imports From Canada
The Trump administration is primed to approve a plan designed to help lower costs of some prescription drugs by allowing states to import them from Canada. The announcement could come before Election Day, and Florida appears to be in line to go first.
KHN’s ‘What the Health?’: It’s Scandal Week
President Donald Trump this week issued a prescription drug pricing order unlikely to lower drug prices, and he contradicted comments by his director of the Centers for Disease Control and Prevention on the need for mask-wearing and predictions for vaccine availability. Meanwhile, scandals erupted at the CDC, the Centers for Medicare & Medicaid Services and the Food and Drug Administration. And the number of people without health insurance grew in 2019, reported the Census Bureau, even while the economy soared. Alice Miranda Ollstein of Politico, Tami Luhby of CNN and Sarah Karlin-Smith of the Pink Sheet join KHN’s Julie Rovner to discuss this and more. Plus, for extra credit, the panelists recommend their favorite health policy stories of the week they think you should read, too.
KHN’s ‘What the Health?’: The Politics of Science
Republicans have all but abandoned the Affordable Care Act as a campaign cudgel, judging from their national convention, at least. Meanwhile, career scientists at the federal government’s preeminent health agencies — the Food and Drug Administration, the Centers for Disease Control and Prevention and the National Institutes of Health — are all coming under increasing political pressure as the pandemic drags on. Joanne Kenen of Politico, Mary Ellen McIntire of CQ Roll Call and Sarah Karlin-Smith of the Pink Sheet join KHN’s Julie Rovner to discuss this and more. Plus, Rovner interviews KHN’s Elizabeth Lawrence about the latest KHN-NPR “Bill of the Month” installment.
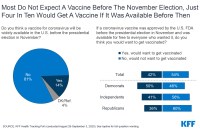
Most Adults Wary of Taking Any Vaccine Approved Before the Election
About 60% of poll respondents are worried that federal regulators will rush to allow a vaccine because of political pressure. Opposition to getting a vaccine that might be authorized before the November election is strongest among Republicans.
Dozens of U.S. Hospitals Poised to Defy FDA’s Directive on COVID Plasma
The FDA, under pressure from the Trump administration, has authorized broader use of convalescent plasma for emergency treatment in COVID patients. But several major hospitals are resisting, saying they’ll opt instead to use the scarce resource to complete a clinical trial.
5 Things to Know About Convalescent Blood Plasma
President Donald Trump touted the Food and Drug Administration’s approval of this unproven COVID-19 treatment for emergency use. That set off reactions ranging from excitement and optimism to scientific concerns and criticism that the decision was politically motivated.
Trump Is Sending Fast, Cheap COVID Tests to Nursing Homes — But There’s a Hitch
Experts say the administration’s approach with antigen tests could add cost and risk for the most vulnerable patients.
Don’t Fall for This Video: Hydroxychloroquine Is Not a COVID-19 Cure
This statement is taken from a video in which a group of doctors air unproven conspiracy theories about the coronavirus. Dr. Immanuel’s claims were among the most inaccurate. And, before it was removed from social media platforms, thee video was viewed millions of times. President Donald Trump retweeted it.
Public Health Experts Fear a Hasty FDA Signoff on Vaccine
The FDA must approve any coronavirus vaccine before it’s widely distributed, but political pressure could cloud the decision.
KHN’s ‘What The Health?’: Trump Twists on Virus Response
President Donald Trump has, for now at least, become a realist on the extent of the COVID-19 crisis around the country, and he is urging Americans to socially distance and wear masks. Meanwhile, on Capitol Hill, Republicans facing a July 31 deadline are scrambling to come together on their version of the next COVID relief bill. Joanne Kenen of Politico, Margot Sanger-Katz of The New York Times and Tami Luhby of CNN join KHN’s Julie Rovner to discuss this and more. Also, Rovner interviews NPR’s Pam Fessler, author of the new book “Carville’s Cure,” which traces the history of the United States’ only federal leprosarium.
Alarma por nuevas etiquetas nutricionales y alergias, en medio de COVID
Ante la escasez de suministros por la pandemia de COVID-19, la FDA elaboró directrices que permiten a los fabricantes sustituir ingredientes sin cambiar las etiquetas de los alimentos.
Pandemic-Inspired Food Labeling Raises Alarms for Those With Food Allergies
The Food and Drug Administration released new “temporary guidance” for manufacturers facing supply chain shortages that allows them to make some ingredient substitutions without changing food labels. The pandemic had already made finding trusted foods difficult for some people with allergies. Now they’re worrying about what’s actually in their go-to products.
How Mis- And Disinformation Campaigns Online Kneecap Coronavirus Response
The pandemic has been marked by a significant amount of misinformation — some spread on purpose — that could prove deadly.