Drugmaker Marathon ‘Pausing’ Delivery Of $89,000-A-Year Muscular Dystrophy Drug
After hearing complaints about its high price, Marathon Pharmaceuticals is pausing the launch of an $89,000 drug for a rare disease.
Grassley Launches Inquiry Into Orphan Drugs After KHN Investigation
Citing a Kaiser Health News investigation, Senate Judiciary Committee Chairman Chuck Grassley vows to examine the orphan drug program and possible fixes.
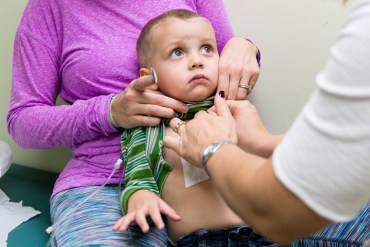
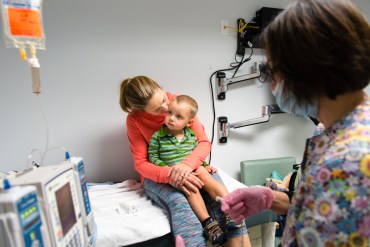
Luke Whitbeck’s life was saved by a rare disease drug, but it costs $300,000 a year.
KHN Video: Orphan Drugs Creating Gold Rush For Pharmaceutical Firms
The designation, which is made by the Food and Drug Administration, allows drugmakers to claim seven years of market exclusivity.
Sky-High Prices For Orphan Drugs Slam American Families And Insurers
Orphan drugs for rare diseases have helped or saved hundreds of thousands of patients like 2-year-old Luke Whitbeck, but families and insurers are picking up the astronomical cost.
Una investigación de Kaiser Health News analiza las acciones de compañias farmacéuticas para manipular los precios de medicamentos huérfanos, utilizados para tratar enfermedades raras.
Drugmakers Manipulate Orphan Drug Rules To Create Prized Monopolies
Drugmakers have brought almost 450 orphan drugs to market and collected rich incentives but nearly a third of those products aren’t new or were repurposed multiple times, an investigation shows.
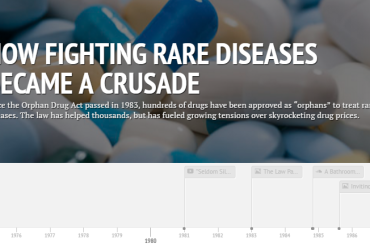
Follow the twists and turns of the orphan drug industry over the past three decades.
Interactive: How Orphan Drugs Win The ‘Monopoly’ Game
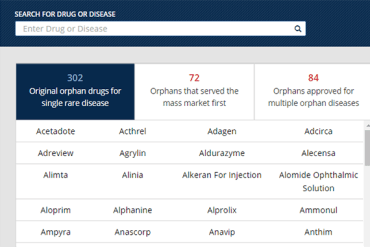
Check out all the drugs the FDA has approved to treat rare diseases. You can search by brand name, or by disease, and see familiar names that were first sold on the mass market or all the drugs that won FDA approval to treat more than one rare disease.
Video: Former U.S. Rep. Henry Waxman Shares Deep Concerns About Orphan Drugs
The former Congressman championed the Orphan Drug Act decades ago but now he fears it’s being manipulated to make money.
A Golden Ticket That Fast-Tracks A Drug Through The FDA
A voucher awarded to companies that find treatments for rare childhood diseases can be sold to the highest bidder — and then used to speed up approvals for much more common drugs.