What the Health? From KFF Health News: Health Funding in Question in a Speaker-Less Congress
A bitterly divided Congress managed to keep the federal government running for several more weeks, while House Republicans struggle — again — to choose a leader. Meanwhile, many people removed from state Medicaid rolls are not finding their way to Affordable Care Act insurance, and a major investigation by The Washington Post attributes the decline in U.S. life expectancy to more than covid-19 and opioids. Lauren Weber of The Washington Post, Victoria Knight of Axios, and Sarah Karlin-Smith of the Pink Sheet join KFF Health News chief Washington correspondent Julie Rovner to discuss these issues and more. Also this week, Rovner interviews physician-author-playwright Samuel Shem about “Our Hospital,” his new novel about the health workforce in the age of covid.
‘I’m So Burned Out’: Fighting to See a Specialist Amplified Pain for Riverside County Woman
Teresa Johnson has been in extreme pain for more than a year after what she believes was a severe allergic reaction to iodine. Her Medi-Cal plan approved her referral to a specialist, but it took her numerous phone calls, multiple complaints, and several months to book an appointment.
In this special encore episode, KFF Health News’ “What the Health?” asks three people who have served as the nation’s top health official: What does a day in the life of the U.S. secretary of Health and Human Services look like? And how much of their agenda is set by the White House? Taped in June before a live audience at Aspen Ideas: Health, part of the Aspen Ideas Festival, in Aspen, Colorado, host and chief Washington correspondent Julie Rovner leads a rare conversation with the current and two former HHS secretaries. Secretary Xavier Becerra and former secretaries Kathleen Sebelius and Alex Azar talk candidly about what it takes to run a department with more than 80,000 employees and a budget larger than those of many countries.
What the Health? From KFF Health News: More Medicaid Messiness
At least 30 states are reinstating coverage for children wrongly removed from the rolls under Medicaid redetermination, the federal government reported. It’s just the latest hiccup in the massive effort to review the eligibility of Medicaid beneficiaries now that the program’s pandemic-era expansion has expired. And federal oversight of the so-called unwinding would be further complicated by an impending government shutdown. Rachel Roubein of The Washington Post, Sandhya Raman of CQ Roll Call, and Sarah Karlin-Smith of Pink Sheet join KFF Health News chief Washington correspondent Julie Rovner to discuss these issues and more. Also this week, Rovner interviews KFF Health News’ Samantha Liss, who reported and wrote the latest KFF Health News-NPR “Bill of the Month” feature, about a hospital bill that followed a deceased patient’s family for more than a year.
As Covid Infections Rise, Nursing Homes Are Still Waiting for Vaccines
“People want covid-19 to be in the rearview mirror,” one nursing home official says. Faced with a slow rollout of the updated covid vaccines, and without state mandates for workers to get vaccinated, most skilled nursing facilities are relying on persuasion to boost vaccination rates among staff and residents.
Florida Gov. Ron DeSantis Injects Presidential Politics Into the Covid Vaccine Debate
Losing ground in the Republican primary, Gov. Ron DeSantis of Florida and his top medical advisers dismissed the recent federal recommendation that almost everyone get an updated covid shot.
Health Workers Warn Loosening Mask Advice in Hospitals Would Harm Patients and Providers
Clinicians, researchers, and workplace safety officers worry new guidelines on face masks from the Centers for Disease Control and Prevention might reduce protection against the coronavirus and other airborne pathogens in hospitals.
A New Covid Booster Is Here. Will Those at Greatest Risk Get It?
The CDC says everyone over 6 months old should get the new covid booster. But the emergency response mechanisms that supported earlier vaccine campaigns are gone. As one expert wonders: How to get boosters to people beyond Democrats, college graduates, and those with high incomes?
What the Health? From KFF Health News: Underinsured Is the New Uninsured
The percentage of working-age adults with health insurance went up and the uninsured rate dropped last year, the U.S. Census Bureau reported this week. There isn’t much suspense about which way the uninsured rate is now trending, as states continue efforts to strip ineligible beneficiaries from their Medicaid rolls. But is the focus on the uninsured obscuring the struggles of the underinsured? Margot Sanger-Katz of The New York Times, Sarah Karlin-Smith of the Pink Sheet, and Joanne Kenen of the Johns Hopkins Bloomberg School of Public Health and Politico join KFF Health News’ Emmarie Huetteman to discuss these issues and more.
Por qué los CDC recomiendan el nuevo refuerzo contra covid para todos
El Comité Asesor sobre Prácticas de Inmunización de los CDC votó 13-1 a favor de la moción después de meses de debate sobre si limitar los refuerzos a grupos de alto riesgo.
Why the CDC Has Recommended New Covid Boosters for All
As covid-19 hospitalizations tick upward with fall approaching, the CDC says it’s time for new boosters — and not only for those at highest risk of serious disease. Here are seven things you need to know.
A pesar de las amplias recomendaciones para el refuerzo anticovid actualizado publicadas el otoño pasado, sólo el 17% de la población la recibió, y alrededor del 43% de las personas de 65 años o más.
Pfizer and Moderna Are Pushing the New Covid Booster. Should You Get It? The CDC Is About to Decide.
Chances are, if you aren’t older, chronically ill, or obese, you don’t need a forthcoming covid vaccine to stay out of the hospital. But it probably wouldn’t hurt.
La publicación fue escrita por el activista anti-vacunas Steve Kirsch, quien ha hecho otras afirmaciones sobre las vacunas que han sido desacreditadas por PolitiFact y otros verificadores de datos.
Activist Misuses Federal Data to Make False Claim That Covid Vaccines Killed 676,000
Anti-vaccine tech entrepreneur Steve Kirsch, whose wild assertions have been repeatedly debunked, wrongly attributes deaths following vaccination to the vaccines themselves. The Centers for Disease Control and Prevention, which runs the database, calls that inaccurate and irresponsible.
What the Health? From KFF Health News: A Not-So-Health-y GOP Debate
The first Republican presidential debate of the 2024 cycle took place without front-runner Donald Trump — and with hardly a mention of health issues save for abortion. Meanwhile, in Florida, patients dropped from the Medicaid program are suing the state for not giving them enough notice or a way to contest their being dropped from the program. Margot Sanger-Katz of The New York Times, Joanne Kenen of the Johns Hopkins Bloomberg School of Public Health and Politico, and Victoria Knight of Axios join KFF Health News’ Julie Rovner to discuss these issues and more. Plus, for “extra credit,” the panelists suggest health policy stories they read this week they think you should read, too.
Few Firm Beliefs and Low Trust: Americans Not Sure What’s True in Age of Health Misinformation
A new poll from KFF shows many Americans aren’t willing to embrace misinformation — but aren’t willing to reject it either. And they don’t know whom to trust.
How a Combination of Covid Lawsuits and Media Coverage Keeps Misinformation Churning
Even as the covid-19 pandemic wanes, litigation — whether about vaccines, masks, or a range of other public health policies made during the pandemic — isn’t about to end.
What does a day in the life of the nation’s top health official really look like? And how much of their agenda is set by the White House? In this special episode of KFF Health News’ “What the Health?” — taped before a live audience at Aspen Ideas: Health, part of the Aspen Ideas Festival, in Aspen, Colorado — host and chief Washington correspondent Julie Rovner leads a rare conversation with the current and two former U.S. secretaries of Health and Human Services. Secretary Xavier Becerra and former secretaries Kathleen Sebelius and Alex Azar talk candidly about what it takes to run a department with more than 80,000 employees and a budget larger than those of many countries.
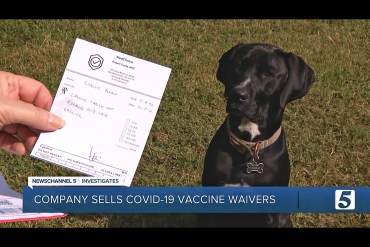
Doctor Lands in the Doghouse After Giving Covid Vaccine Waivers Too Freely
Richard Coble issued vaccine waivers to patients in at least three states without examining them. He was exposed by a Nashville TV station that bought a waiver for a Labrador retriever named Charlie.